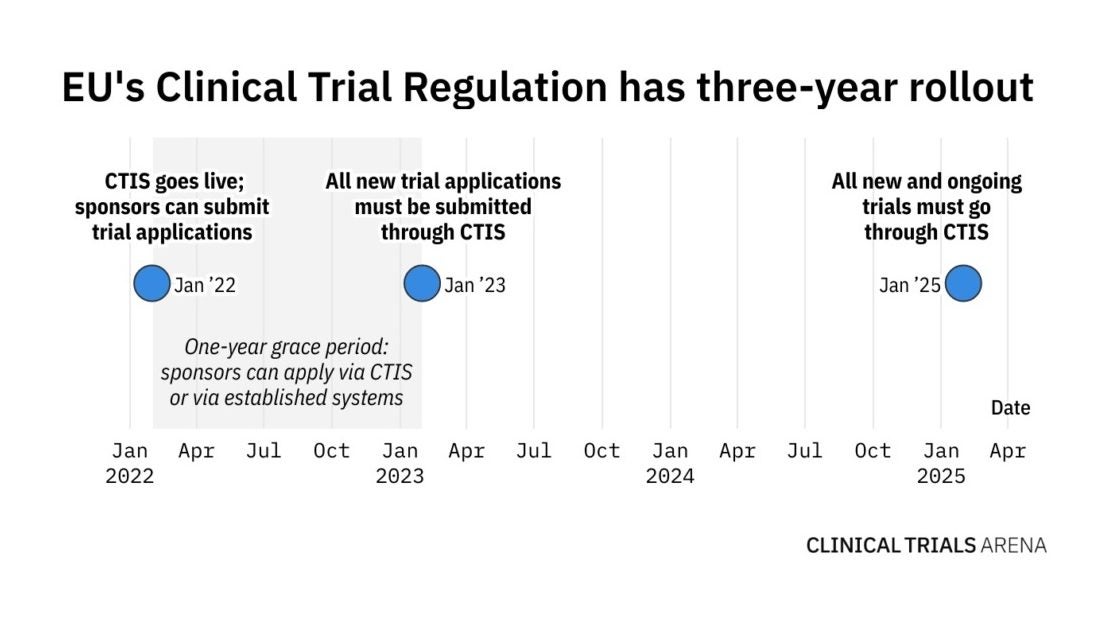
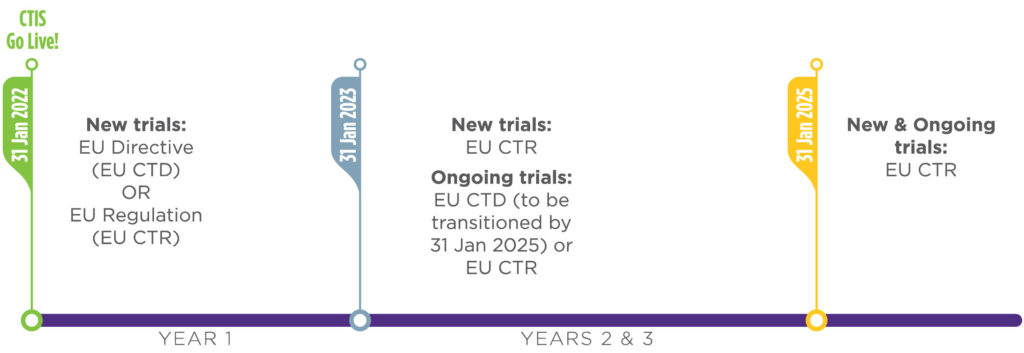
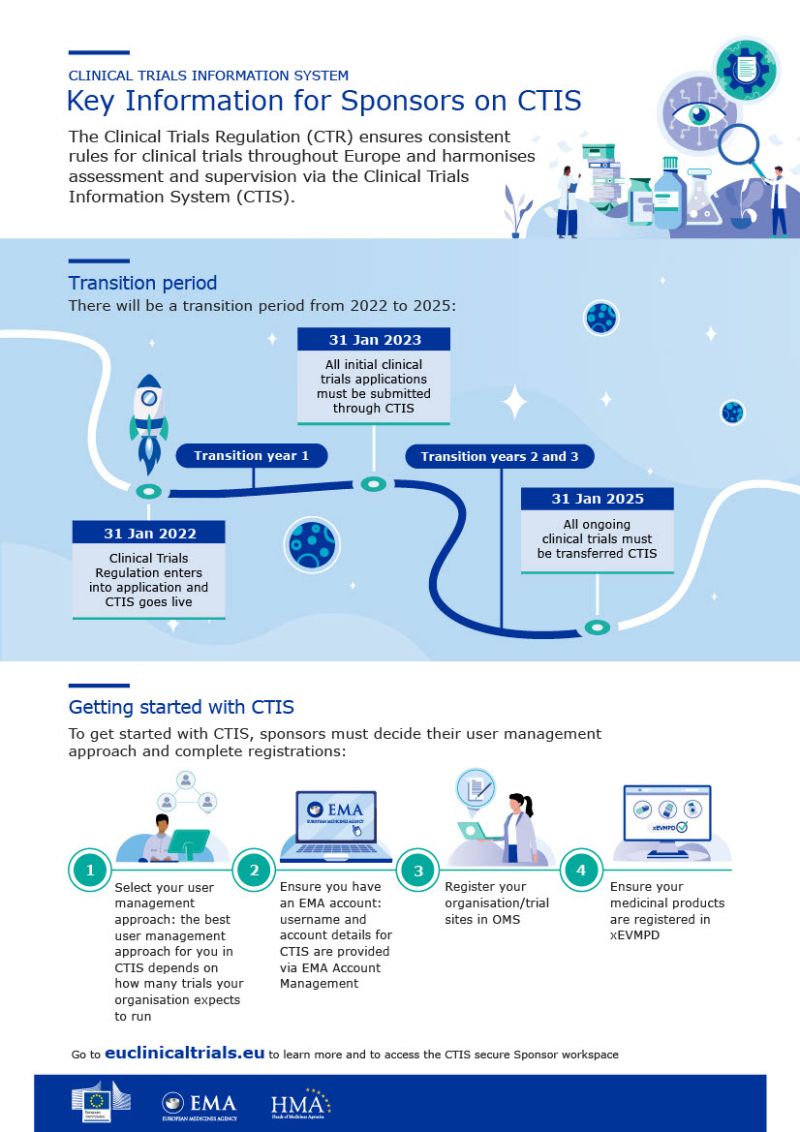
Webinaire Clinical Trials Information System (CTIS): Préparation à l'utilisation obligatoire de la Régulation des Essais Cliniques à compter du 31/01/2023

CTIS (Clinical Trials Information System). Arzt zeigt auf digitales medizinisches Interface. Text umgeben von Icons, angeordnet im Kreis. Photos | Adobe Stock