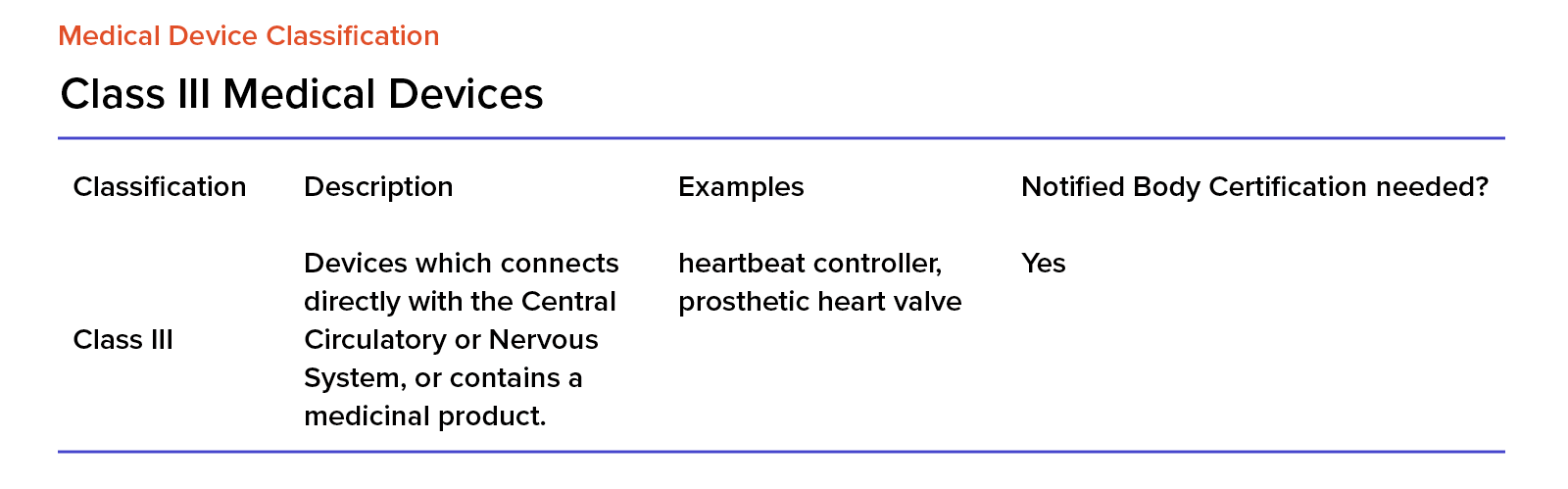
MDR - Un système embarqué polyvalent pour l'acquisition, le traitement, l'enregistrement et la diffusion de données | Safran

MDR - Un système embarqué polyvalent pour l'acquisition, le traitement, l'enregistrement et la diffusion de données | Safran

MDR - Un système embarqué polyvalent pour l'acquisition, le traitement, l'enregistrement et la diffusion de données | Safran
Vecteur Stock MDR - Medical Device Regulation Concept With icons. Cartoon Vector People Illustration | Adobe Stock

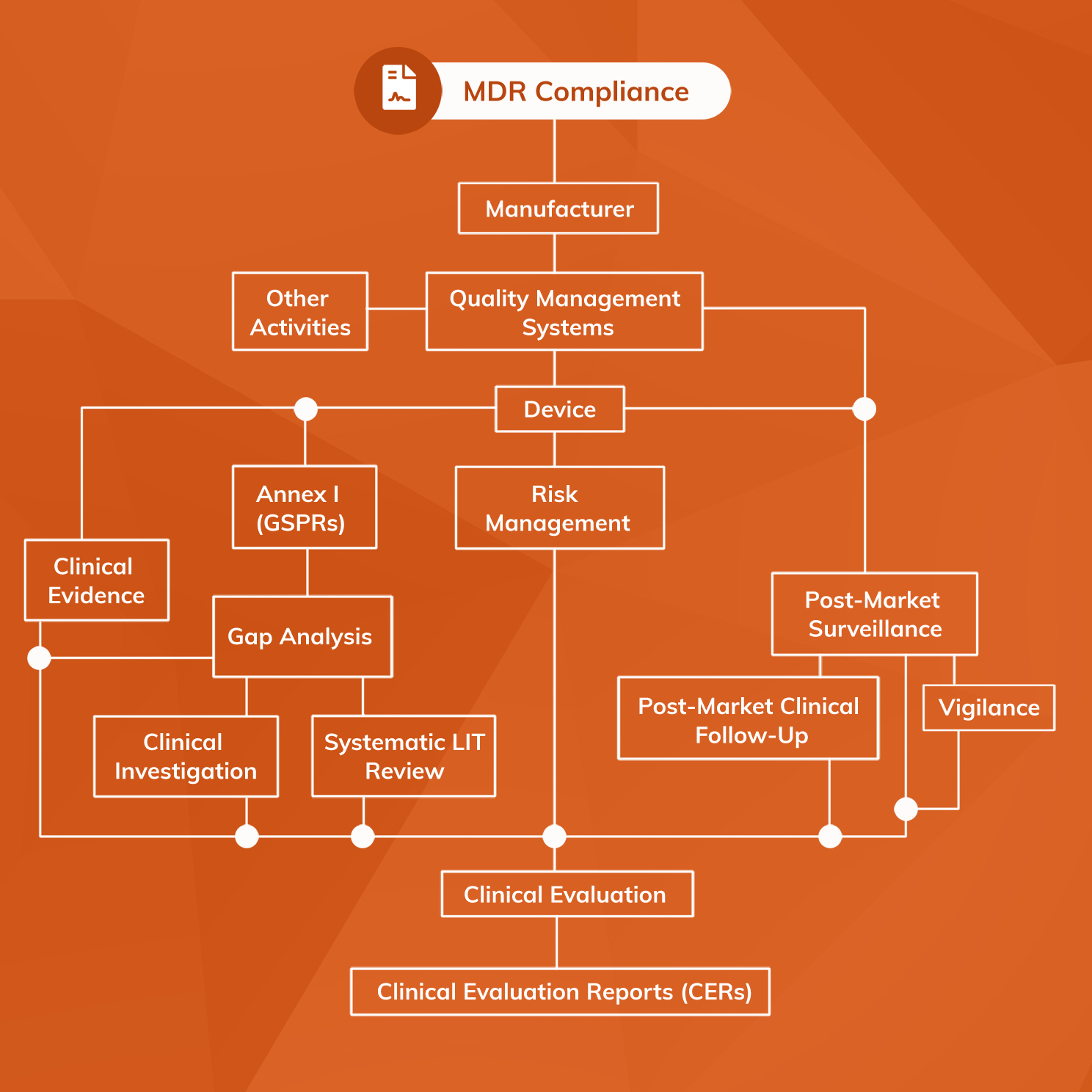
Combined audits MDD/AIMDD and MDR : requirements and impact on the quality management system - GMED Medical Device Certification




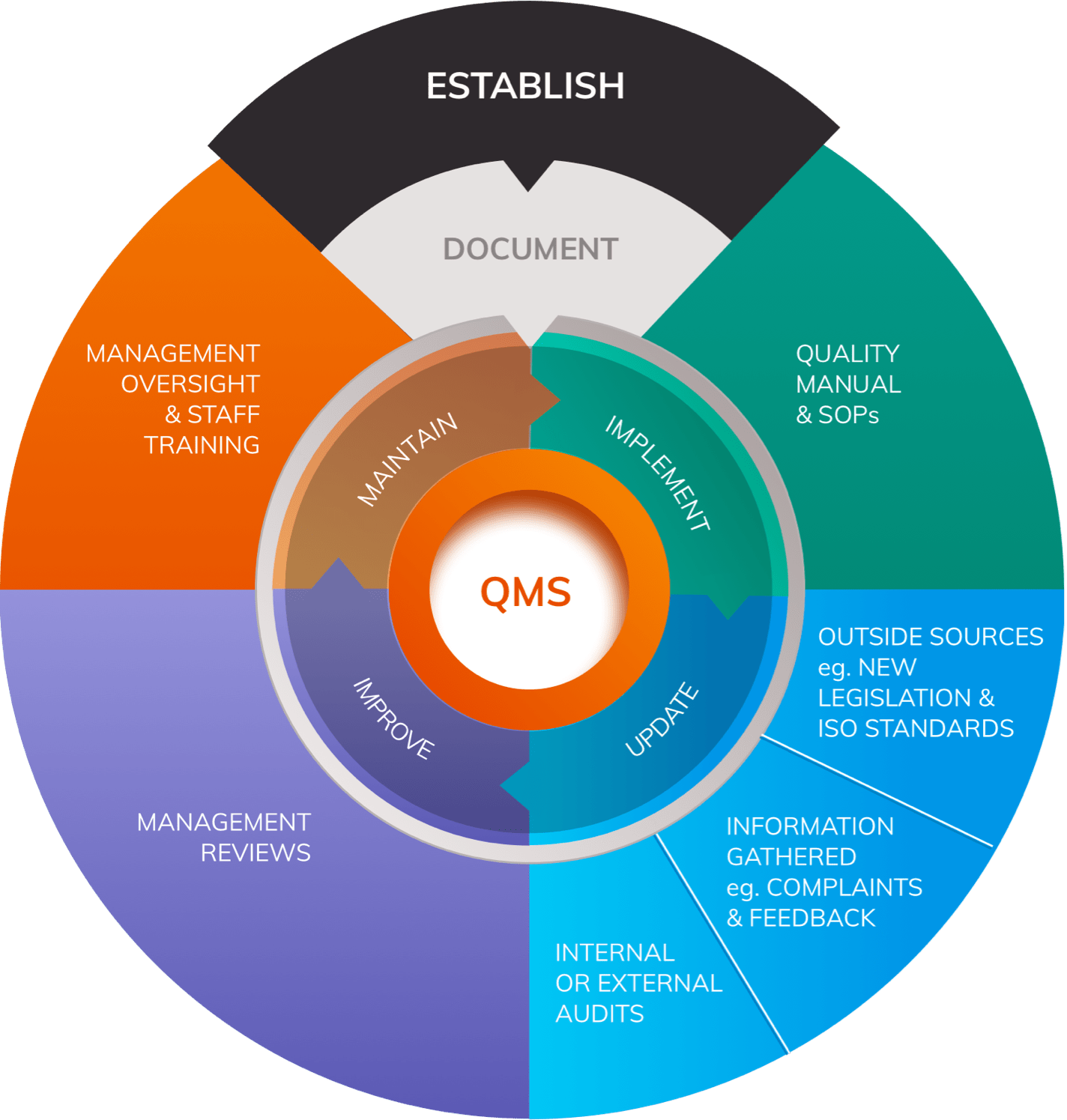

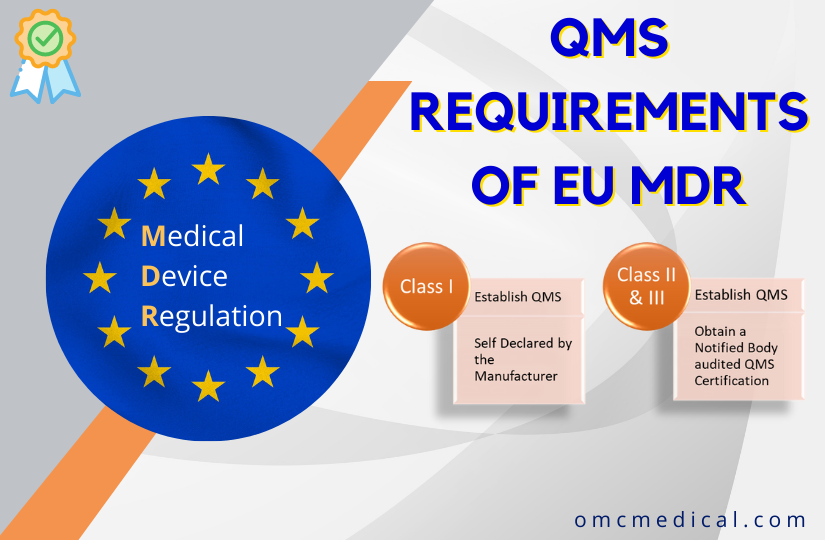



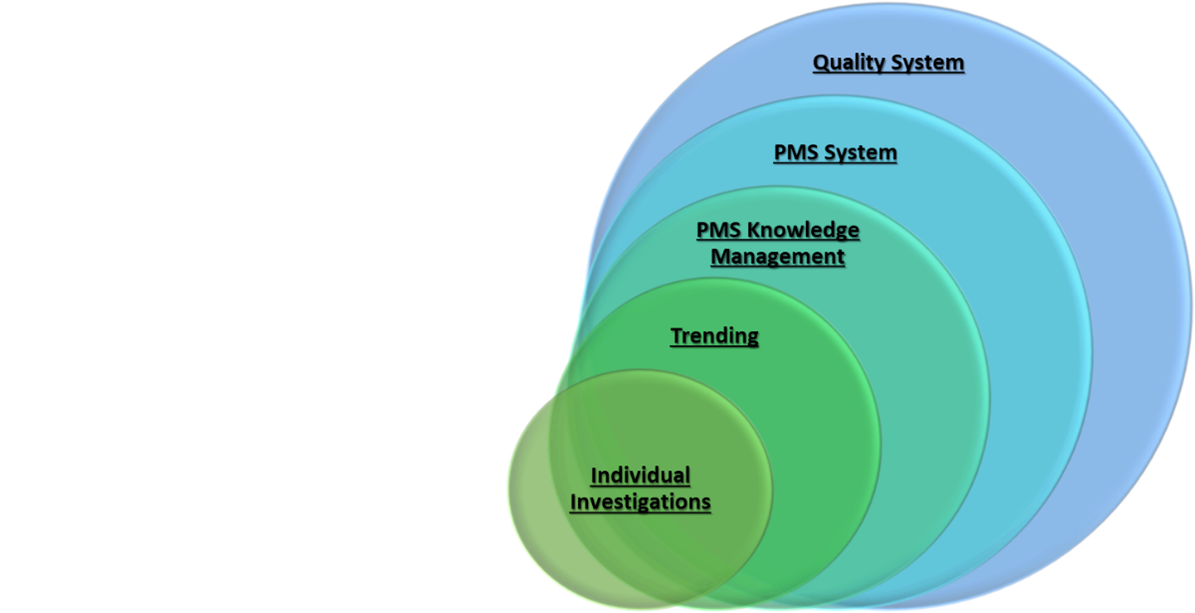

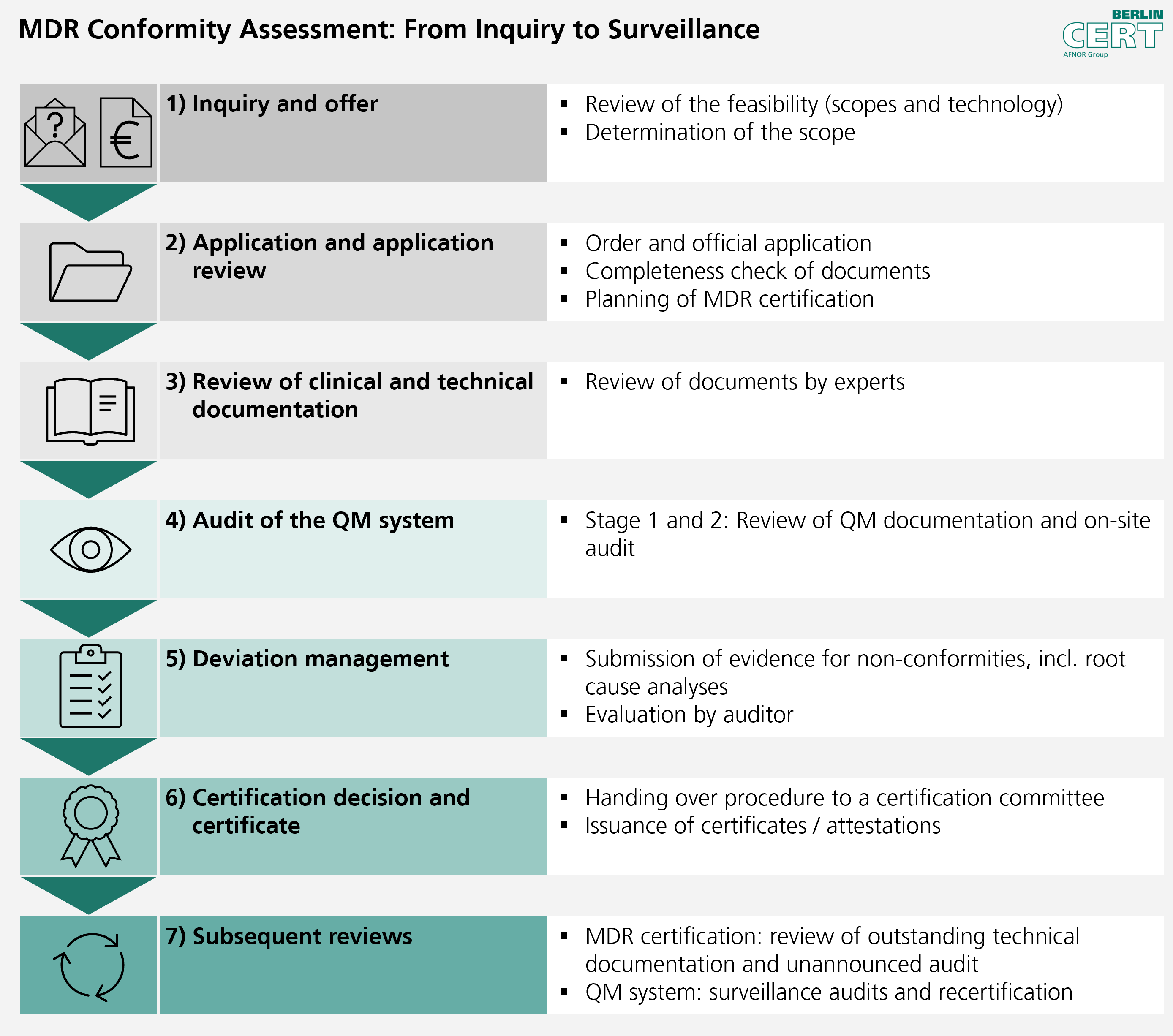

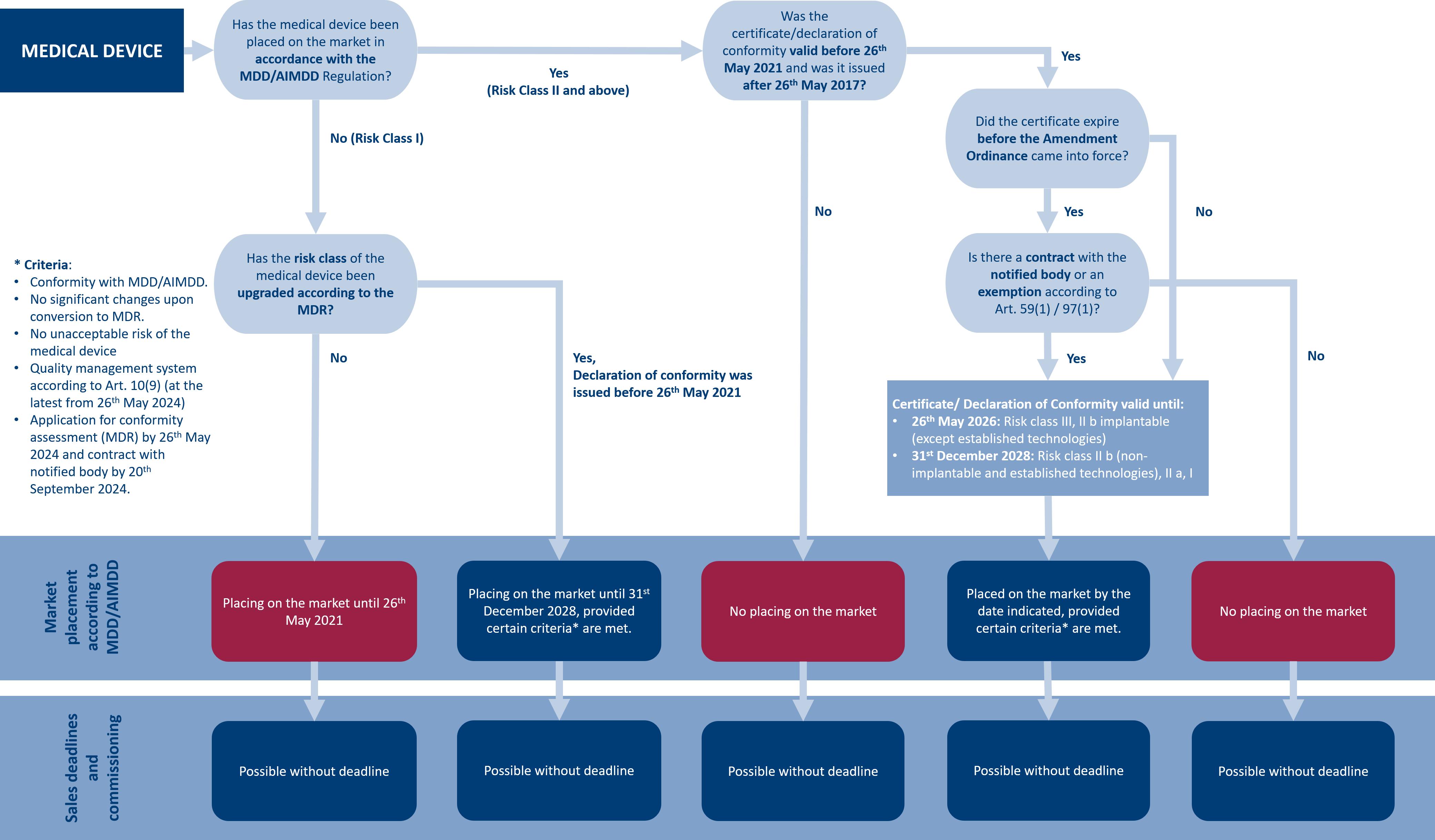

![How System and Procedure Pack are regulated under EU MDR? [Erik Vollebregt] - YouTube How System and Procedure Pack are regulated under EU MDR? [Erik Vollebregt] - YouTube](https://i.ytimg.com/vi/HoqcVBAr960/maxresdefault.jpg)